
Three health products have been found to contain undeclared western medicinal ingredients which can cause serious adverse reactions, The Health Sciences Authority (HSA) warned on Monday (Jan 25).
HSA added that all three products were either purchased overseas through friends or from online platforms.
They did not contain a list of ingredients on their labels, which complementary health products are required to carry.
‘Huo Xue Qing Gan Jie Du Wan’ and ‘Huang Niu Mu Shi Ni De Jiu Xing’ were purchased overseas and claimed to be able to treat various forms of chronic ailments, such as arthritis, liver disorders, high blood pressure, diabetes and stroke.
However, one patient in his 40s was hospitalised for Cushing’s syndrome – a condition characterised by round face or ‘moon face’ and upper body obesity with thin limbs – joint pain, weight gain and insomnia after consuming the ‘Huang Niu Mu Shi Ni De Jiu Xing’.
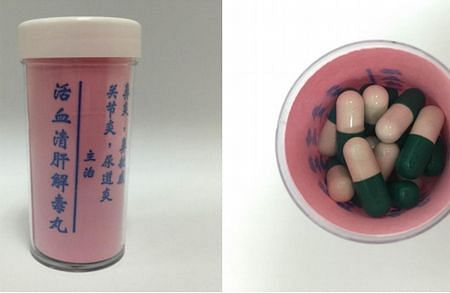
1. Huo Xue Qing Gan Jie Du Wan

2. Huang Niu Mu Shi Ni De Jiu Xing
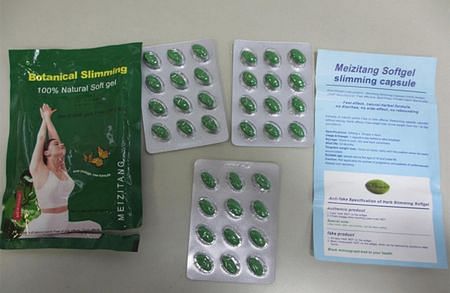
3. Meizitang Botanical Slimming 100% Natural Soft Gel
The undeclared drug ingredients found are dexamethasone, chlorpheniramine and diclofenac.
Dexamethasone is a potent steroid usually prescribed for inflammatory conditions, which should only be used under strict medical supervision. Long-term unsupervised use of oral steroids can cause Cushing’s syndrome, increased blood glucose levels leading to diabetes, high blood pressure, cataracts, muscular and bone disorders, and an increased risk of infections.
Chlorpheniramine is an antihistamine used to relieve allergic reactions such as rhinitis, and can only be used under medical supervision by a pharmacist or doctor. Adverse effects from the use of chlorpheniramine include drowsiness, blurred vision, vomiting, constipation and poor coordination.
Diclofenac is a potent painkiller and may potentially cause serious gastric bleeding, as well as cardiovascular events such as heart attacks and stroke when used for a prolonged period. It should be used under close medical supervision, especially in patients with underlying heart conditions.
Members of the public who have consumed these products are advised to consult a doctor as soon as possible and stop taking them immediately.
Sellers of these products should cease all sales and distribution immediately . If convicted, offenders face a fine of up to $10,000 or two-years jail under the Poisons Act.
This story was originally published in AsiaOne.com. For more stories like this, head to www.asiaone.com/women.

